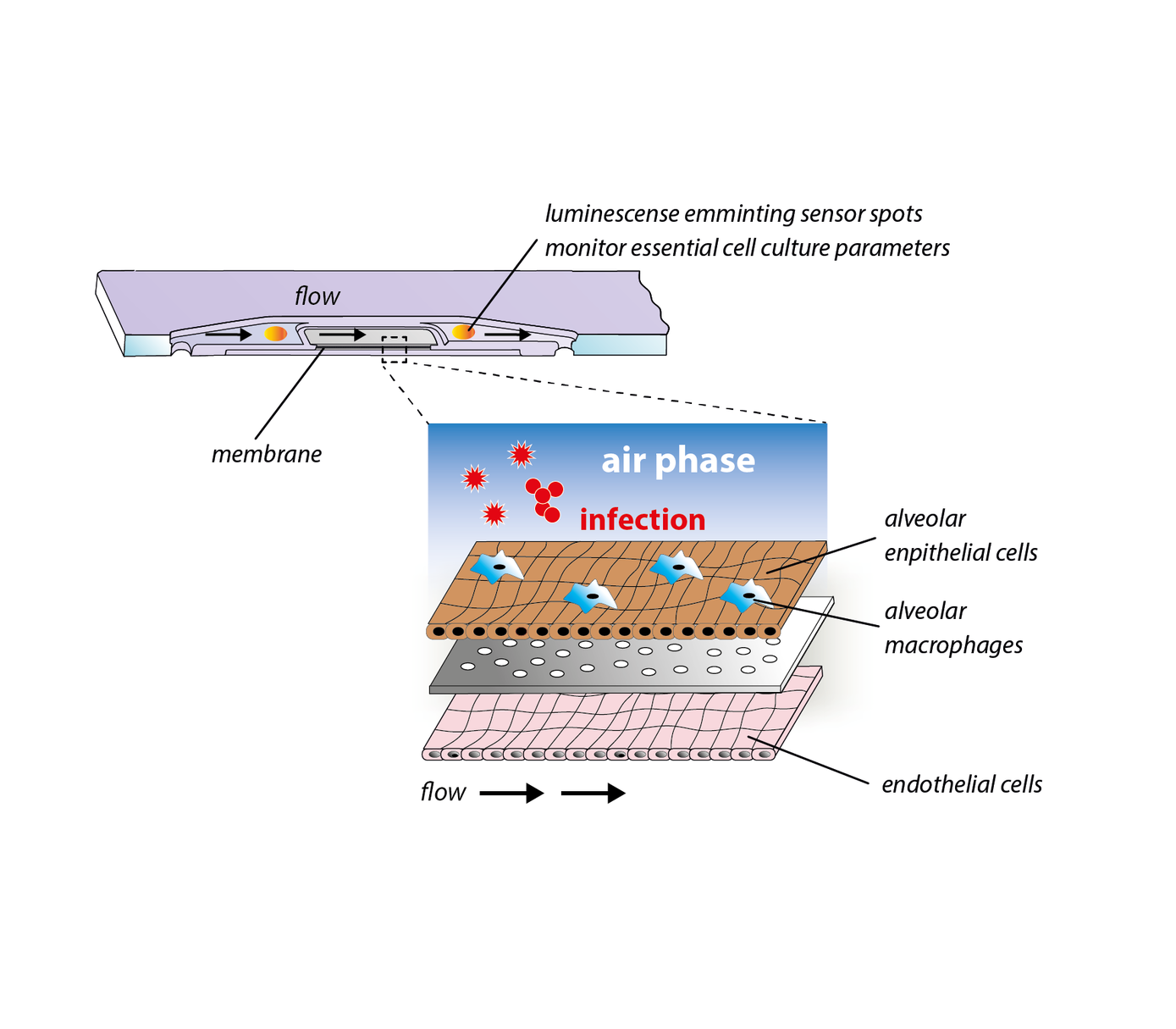
Lung-on-chip
The lung is the central organ for the exchange of gas in the human body. It is characterized by a large surface area in relation to its volume and comprises the distal branching of the trachea, bronchial tubes, bronchioles and alveoli.
Increased exposure of the lungs to microbial pathogens can lead to pneumonia, lesion of the lung tissue (acute lung injury, ALI) and to a acute respiratory distress syndrome (ARDS). The major risk factor of ALI or ARDS is severe sepsis in 79% of cases, which may be of pulmonary (46%) or non-pulmonary (33%) origin. Pneumonia is the second most commonly acquired infectious disease worldwide with the highest population-based mortality rate. Murine models for bacterial and/or viral infections have several limitations, e.g. transmission of pathogens is inefficient in adult mice compared to the human situation. On the other hand, conventional human 2D mono-cell-culture models of the alveolus often fail to maintain differentiation and expression of alveolus-specific functions.
To close this gap, we have developed a immunocompetent lung-on-chip model that comprises human alveolar endothelial and epithelial cells as well as surrogates of alveolar macrophages. The model continuously produces surfactant and maintains an air-liquid-interphase for several weeks. It is able to replicate essential processes of bacterial and viral lung infections in vitro and represents a powerful platform for investigations on host-pathogen interaction and the identification of molecular and cellular targets for novel treatment strategies of pneumonia.