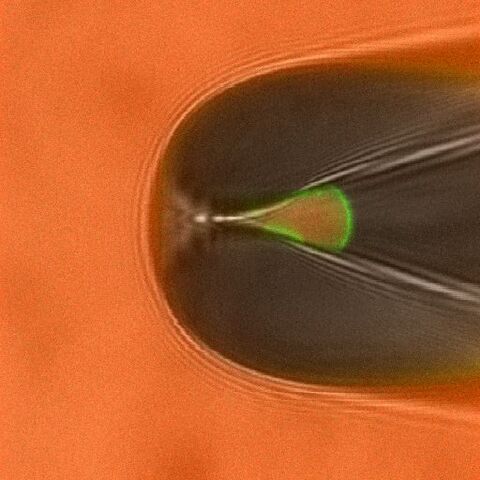
By combining electrophysiological and optical techniques the gating of ion channels can be correlated to other events such as the binding of fluorescently labeled ligands or conformational changes.
In a joint project with Dr. Vasilica Nache (Institute for Physiology II) within the CRC-TR ReceptorLight this technique will be used to investigate the binding of cyclic nucleotides to CNG channels.
The technique will also be applied to glutamate receptors.
Researchers involved in the project
Collaborations
- Prof. Dr. Klaus Benndorf, Institute of Physiology II, Jena University Hospital
Dr. Jana Kusch, Institute of Physiology II, Jena University Hospital
Dr. Vasilica Nache, Institute of Physiology II, Jena University Hospital
Founding
- Deutsche Forschungsgemeinschaft / German Research Foundation
(Projekt B1 SFB-TR166 ReceptorLight)
Selected publications
- Biskup C., Kusch J., Schulz E., Nache V., Schwede F, Lehmann F., Hagen V., Benndorf K.:
Relating ligand binding to activation gating in CNGA2 channels.
Nature 446, 440-443 (2007). - Kusch J., Biskup C., Thon S., Schulz E., Nache V., Zimmer T., Schwede F., Benndorf K.:
Interdependence of receptor activation and ligand binding in HCN2 pacemaker channels.
Neuron 67, 75-85 (2010). - Kusch J., Thon S., Schulz E., Biskup C., Nache V., Zimmer T., Seifert R., Schwede F., Benndorf K.:
How subunits cooperate in cAMP-induced activation of homotetrameric HCN2 channels.
Nat. Chem. Biol. 8, 162-169 (2011). - Nache V., Zimmer T., Wongsamitkul N., Schmauder R., Kusch J., Reinhardt L., Bönigk W., Seifert R., Biskup C., Schwede F., Benndorf K.:
Differential regulation by cyclic nucleotides of the CNGA4 and CNGB1b subunits in olfactory cyclic nucleotide-gated channels.
Sci Signal. 5, 232 (2012).