Background
Allergic diseases of the immediate type such as allergic Asthma bronchiale are characterized by elevated serum levels of IgE, infiltration of the affected tissue by inflammatory cells, and a Th2-dominated type of immune reaction. Dysregulation of cytokine function is crucial for both acute and chronic symptoms of the disorder. We have recently concentrated on two cytokines which both signal via heterodimeric cytokine receptors and JAK/STAT pathways, and play central roles in Asthma: Interleukin-13 and Thymic Stromal Lymphopoietin (TSLP).
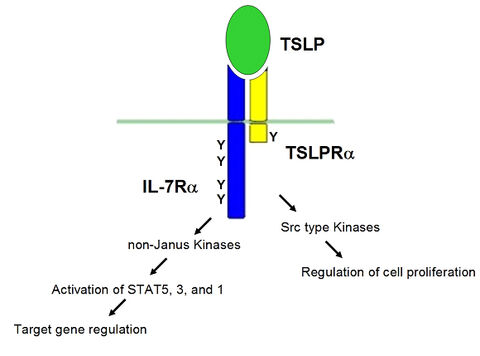
Figure A
IL-13 is a cytokine with multiple functions in normal immune processes and inflammatory diseases. It is mainly produced by activated T cells, but also by various other cell types such as mast cells, basophils, eosinophils, and dendritic cells. IL-13 is structurally and functionally closely related to the prime Th2 cytokine IL-4 and shares its property to induce immunoglobulin class switching to IgE. However, it has specific critical functions in the development of allergic asthma and its symptoms (hyperresponsiveness to specific antigens, elevated IgE levels, mucus hypersecretion and subepithelial fibrosis).
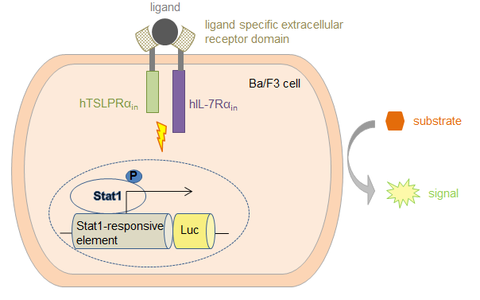
TSLP is an interleukin-7-like cytokine which emerged in the last years as a central player in the development of allergic symptoms, especially in the airways, and is a prime regulatory cytokine at the interface of virus- or antigen-exposed epithelial cells and dendritic cells. Since TSLP links contact of allergen with the airway epithelium to the onset and maintainance of the asthmatic syndrome, defining the signal transduction underlying TSLP expression and function is of profound interest for a better understanding of the disease and for the development of new therapeutics. The TSLP receptor consists of a specific TSLPR a-chain and the IL-7 receptor a-chain. Upon ligand-induced activation, it activates JAK/STAT pathways and influences cell proliferation as well as specific gene regulation (see Fig. A).