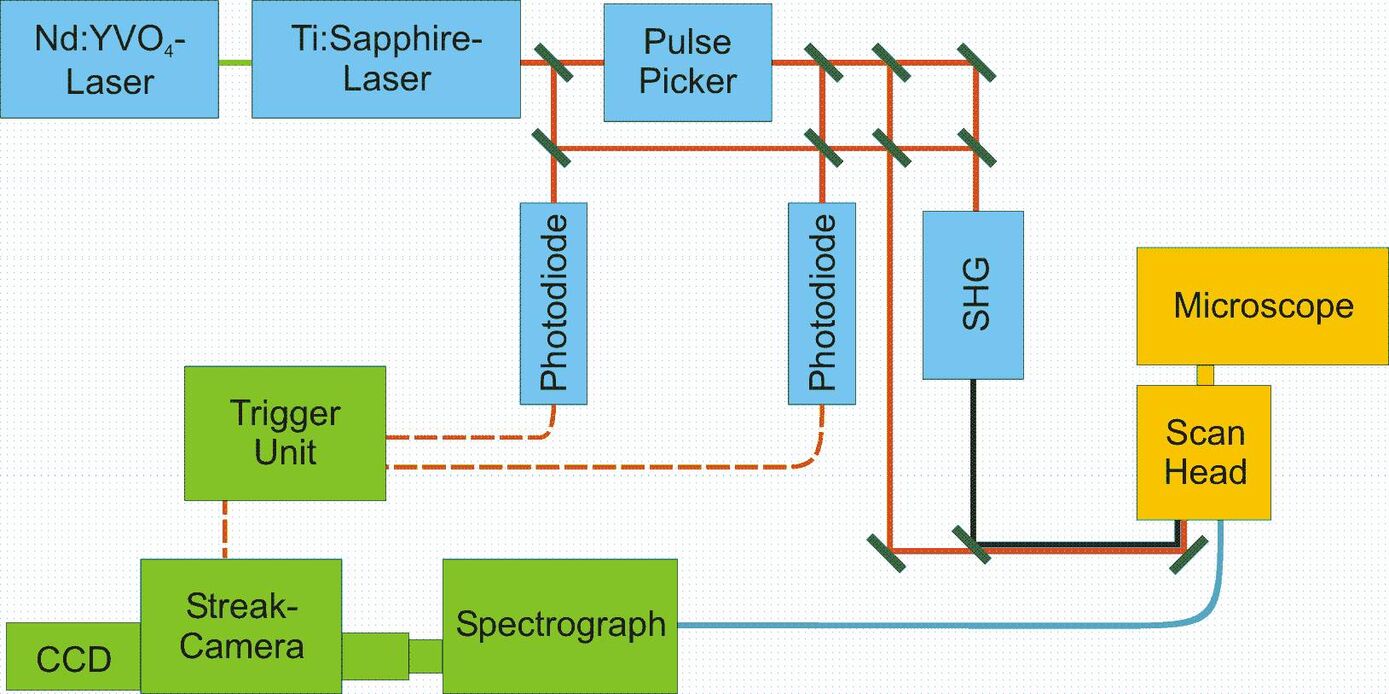
Beam path
In order to be able to acquire time-resolved fluorescence spectra from cellular compartments of living cells, we adapted a spectrograph and a streak camera to a confocal laser scanning microscope (LSM 510, Carl Zeiss GmbH, Göttingen, Germany). For fluorescence excitation we used a femtosecond Ti:Sapphire laser, whose repetition rate is decreased to 2 MHz by a pulse picker (Fig. 1).
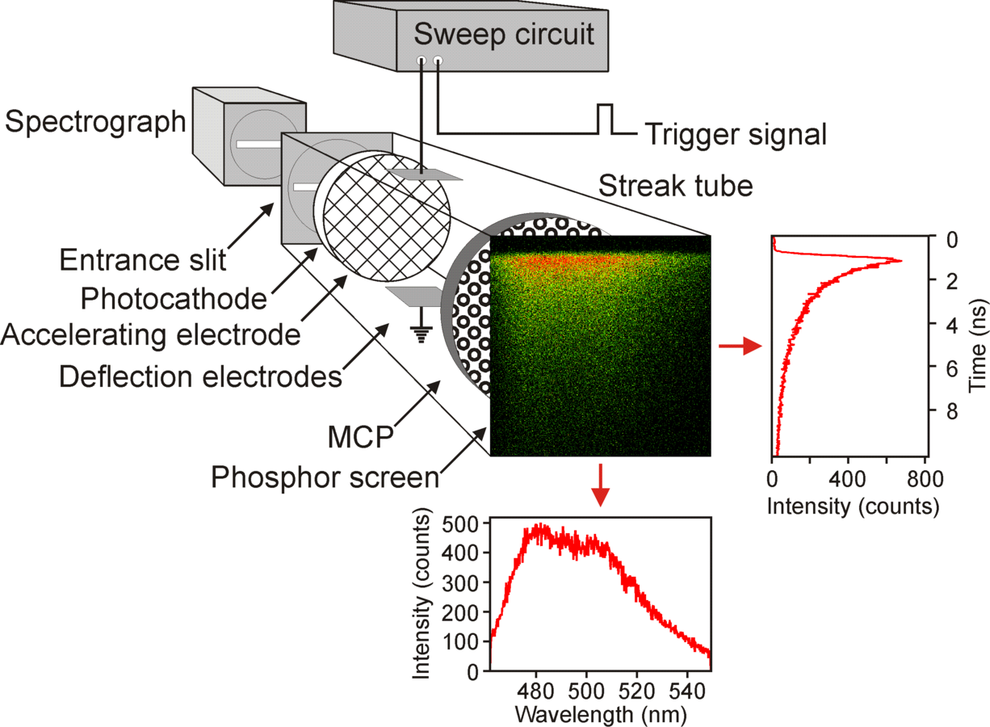
Figure 1: Beam path. For pulsed excitation of the sample a Ti:Sapphire laser (Mira 900, Coherent), pumped by a frequency doubled NdYVO4-laser (Verdi 5 W, Coherent) is used. A pulse picker decreases the repetition rate to 2 MHz. The frequency of the laser light can be doubled by a BBO crystal. Fluorescence emitted by the sample is guided to a spectrograph (250is, Chromex Inc., Albuquerque) and a Hamamatsu streak camera (C5680, with M5677 sweep unit). (Fig. reproduced from: Biskup et al., Nat. Biotechnol. 22, 220-224 (2004))