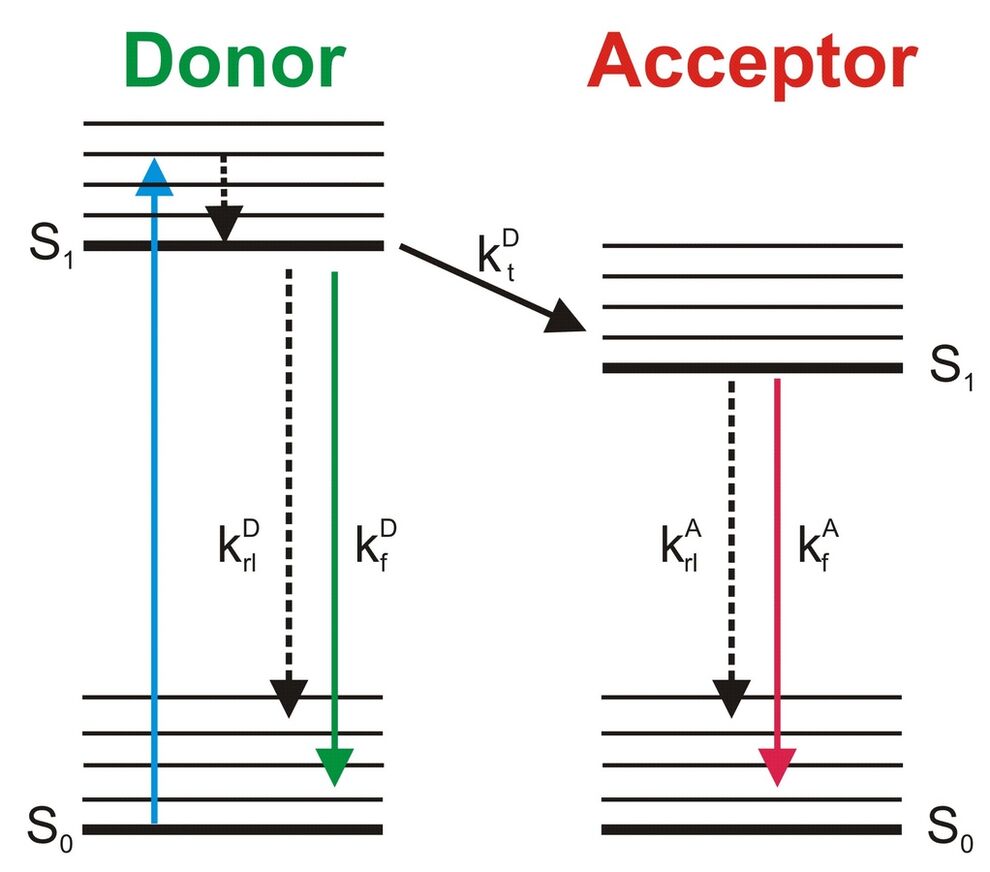
Determination of the FRET efficiency via fluorescence lifetime measurements
Measurements of fluorescence lifetimes have the advantage of being independent of the respective concentrations of donor and acceptor molecules. Thus, control measurements and FRET measurements can be carried out in different cells.
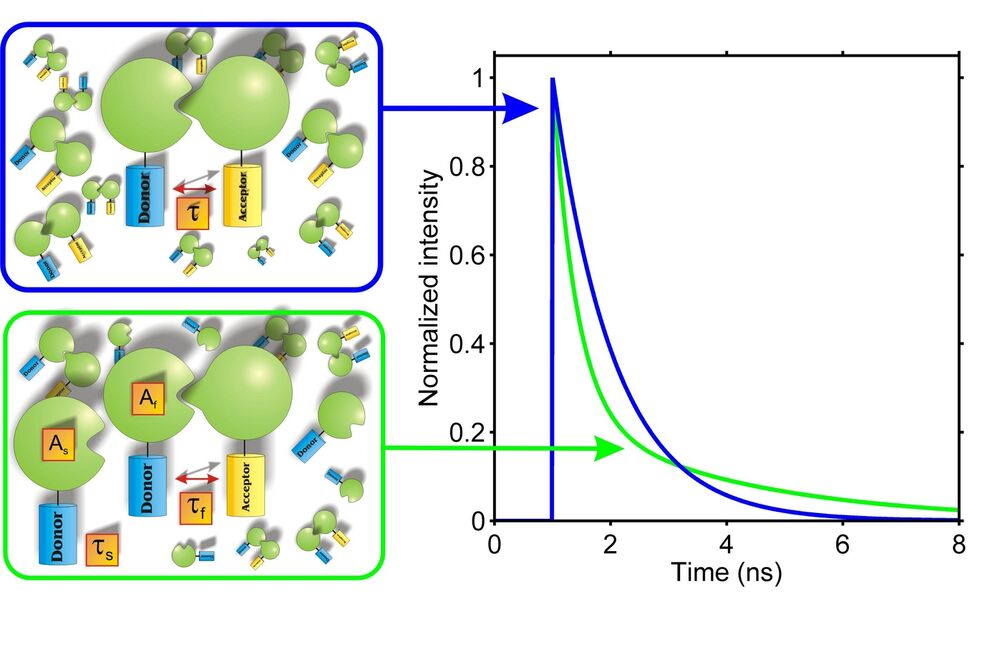
Moreover, lifetime-based FRET measurements can also provide additional information about the interacting partners, which is not accessible via intensity-based measurements. This is demonstrated in Fig. 2, which considers two cases of interacting proteins labeled with appropriate fluorophores. In the first case (left, upper panel) it is assumed that all donor molecules are associated with an acceptor molecule. In this case only one class of donor molecules exists and the resulting fluorescence decay is monoexponential. In a case, however, where only a part of the donor molecules is associated (left, lower panel) two populations of donor molecules – bound and unbound – are present, giving rise to a biexponential decay. By measuring and analyzing the fluorescence decay one can not only distinguish between the two cases but even get an estimate for the fraction of bound and unbound molecules. In intensity measurements, however, only the integral of the fluorescence decay is measured and all the precious information provided by the decay kinetics is lost.
The fraction of interacting and non-interacting donor molecules can be determined by fitting a biexponential function to the fluorescence decay. The slow lifetime component (τs) corresponds to the lifetime of the unbound (and unquenched) donor molecules, whereas the fast lifetime (τf) is a function of the distance and orientation of the donor- und acceptor fluorophores. The relative amplitudes (As, Af) correspond to the contributions of bound and unbound donor molecules to the total fluorescence decay recorded from the sample.
Applications
The technique has been employed in numerous studies, some of which are elucidated on the following pages:
- Association of ion channel subunits
- Structure and dynamic of the postsynaptic density
- Intracellularsignal transduction pathways
Further developments
Despite the advantage of fluorescence lifetime based FRET measurements discussed above, many errors can occur during a measurement. For instance, the molecules used to label proteins can photobleach or photoconvert. In this case the fluorescence lifetime can decrease (Hoffmann et al., J. Biomed. Opt. 13, 031205 (2008)). Without additional controls such a decrease in the donor fluorescence lifetime can be erroneously interpreted as FRET. Such a control can be achieved via spectrally-resolved fluorescence lifetime measurements. In our group several techniques to measure spectrally resolved fluorescence decays in the time domain are established (see Methods).
Researchers involved in the project
Project related publications
- Biskup C., Zimmer T., Benndorf K.:
FRET between cardiac Na+ channel subunits measured with a confocal microscope and a streak camera.
Nature Biotechnol. 22, 220-224 (2004).
- Biskup C., Kelbauskas L., Zimmer T., Benndorf K., Bergmann A., Becker W., Ruppersberg J.P., Stockklausner C., Klöcker N.:
Interaction of PSD-95 with potassium channels visualized by fluorescence lifetime-based resonance energy transfer imaging.
J. Biomed. Opt. 9, 753-759 (2004).
- Biskup C., Böhmer A., Pusch R., Kelbauskas L., Gorshokov A., Majoul I., Lindenau J., Benndorf K., Böhmer F.D.:
Visualization of SHP-1 target interaction
J. Cell Sci. 177, 5165-5178 (2004).
- Biskup, C., Zimmer, T., Kelbauskas, L., Hoffmann, B., Klöcker, N., Becker, W., Bergmann, A., Benndorf, K.:
Multi-dimensional fluorescence lifetime and FRET measurements.
Microsc. Res. Tech. 70, 442-451 (2007).
- Shen Q.H., Saijo Y., Mauch S., Biskup C., Bieri S., Keller B., Seki H., Ülker B., Somssich I., Schulze-Lefert P.:
Nuclear activity of MLA immune receptors links isolate-specific and basal resistance responses.
Science 315, 1098-1103 (2007).
- Orthaus S., Biskup C., Hoffmann B., Hoischen C., Ohndorf S., Benndorf K., Diekmann S.:
Assembly of the inner kinetochore proteins CENP-A and CENP-B in living human cells.
ChemBioChem 9, 77-92 (2007).
- Hoffmann B., Zimmer T., Klöcker N., Kelbauskas L., König K., Benndorf K., Biskup C.:
Prolonged irradiation of ECFP and Cerulean can invalidate FRET measurements.
J. Biomed. Opt. 13, 031205 (2008).
- Kwaaitaal M., Keinath N.F., Pajonk S., Biskup C., Panstruga R.:
Combined bimolecular fluorescence complementation and Forster resonance energy transfer reveals ternary SNARE complex formation in living plant cells.
Plant Physiol. 152,1135-1147 (2010).
- Hoffmann B., Klöcker N., Benndorf K., Biskup C.:
Visualization of the dynamics of PSD-95 and Kir2.1 interaction by fluorescence lifetime-based resonance energy transfer imaging.
Medical Photonics 27, 70-82 (2015).