We developed a framework of instrumentation, experimental design and data-evaluation to maximize the information gained in macro-patch concentration clamp experiments. It is based on three components:
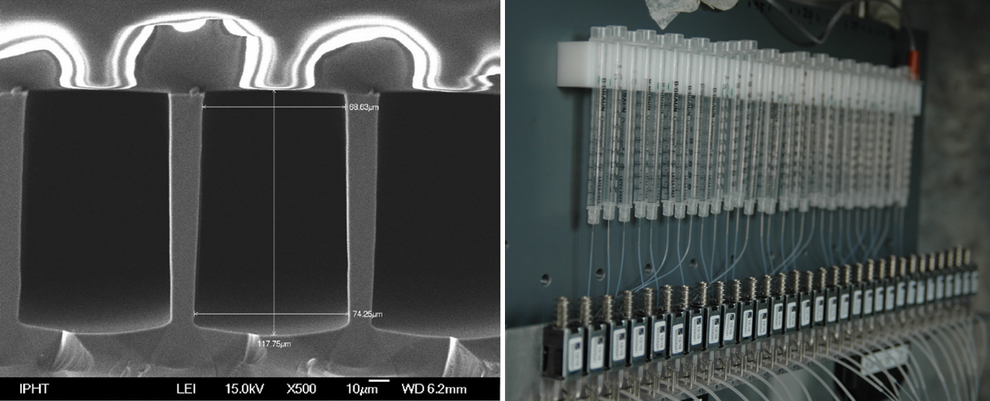
- A fast, piezo-driven 30-channel solution applicator. Its microfluidic structure assumes minute reagent consumption.
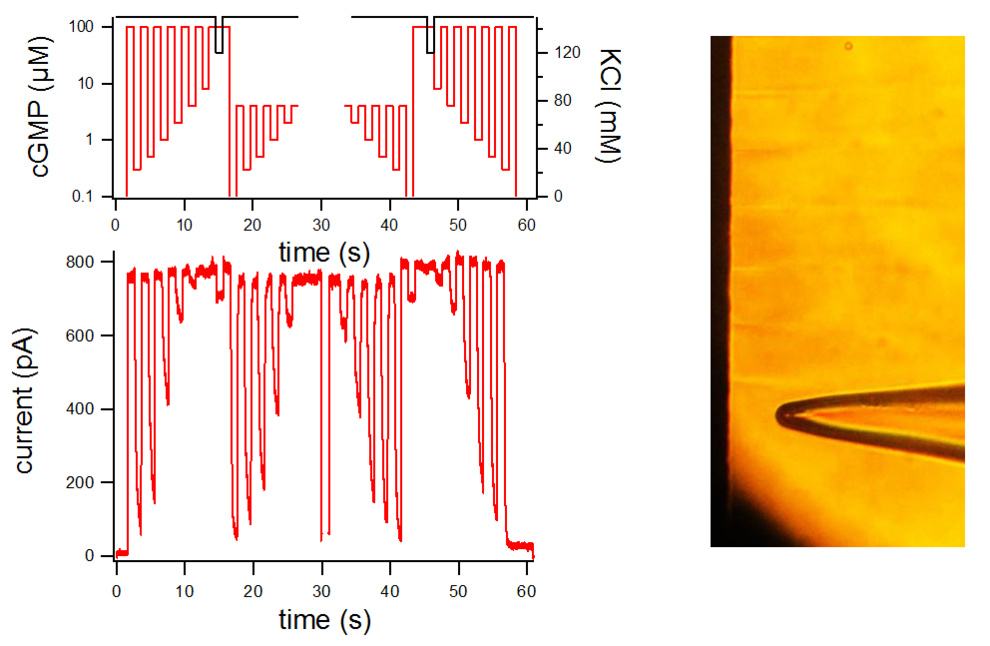
- Direct recording of the instrument response e.g. solution exchange/diffusion during the experiment
- Explicit analytical treatment of diffusion processes in the patch, allowing to determine time constants faster than the solution exchange.