Module IV/1 - Generation and analysis of transgenic mouse models

Since the first gene transfers into mice in 1980, transgenic mice have allowed researchers to study the role of genes in development, physiology and disease. In this module participants will have the opportunity to get an introduction into current technical possibilities (Figure Purkinje-cell specific disruption of Kcc2). In this module several mouse models for hereditary human disorders from our currently ongoing scientific projects will be presented. Practical work will include molecular biology including cloning strategies and genotyping techniques. You will also have the possibility to get insights into mouse embryo manipulation. Selected initial mouse phenotyping techniques will be demonstrated including morphological techniques, behavioral analysis, and electrophysiology.
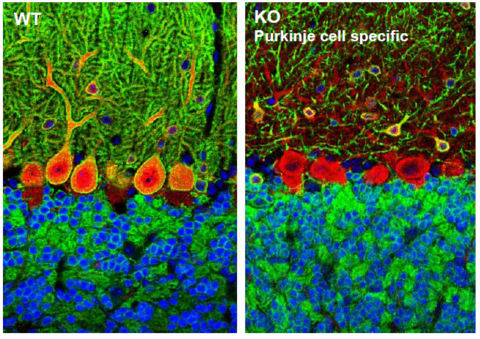
Staining of cerebellar sections from wild-type (WT, left) and Purkinje cell specific Kcc2 knockout mice (KO, right). Kcc2 is stained in green, Purkinje cells are labelled in red for calbindin and nuclei are stained with DAPI in blue. In the cell specific KO Purkinje cells do not express Kcc2 any more, while Kcc2 expression in granule neurons and interneurons is preserved.
Module IV/2 - Molecular Medicine of Life-Threatening Infections

Department of Anesthesiology and Intensive Care Medicine
Pneumolysin (PLY) is a major virulence factor of Streptococcus pneumoniae and crucial for the pathogenesis of invasive pneumococcal disease and sepsis following community-acquired pneumonia (CAP). The β-pore-forming toxin recognizes membrane cholesterol leading to disintegration of the alveolar epithelial barrier and impairment of innate immunity. Remotely, pneumococcal CAP triggers a hepatic activation of the sterol biosynthesis through a yet unknown mechanism that depends on PLY in mice. Supplementation of cholesterol species or delivery of cholesterol by polymer nanoparticles is evaluated as a candidate for adjuvant therapy to counteract pore formation. PLY may be seen as a homeostasis-altering molecular pattern (HAMP) as it directly activates key regulators of the lipid homeostasis resulting in a rapid increase of cellular cholesterol. Some of these effects may be the result of inflammasome-signaling activated through PLY-pore stress. Inflammasome signaling partly contributes to cell death in pneumococcal diseases but on the other hand may also alter the lipid homeostasis, increasing lipid and particular cholesterol biosynthesis. Together we will investigate the nature of the PLY-activated sterol biosynthesis to decipher its relevance in the pathophysiology and survival of life-threatening infections applying molecular and functional assays on cell lines stimulated with bacterial toxines.
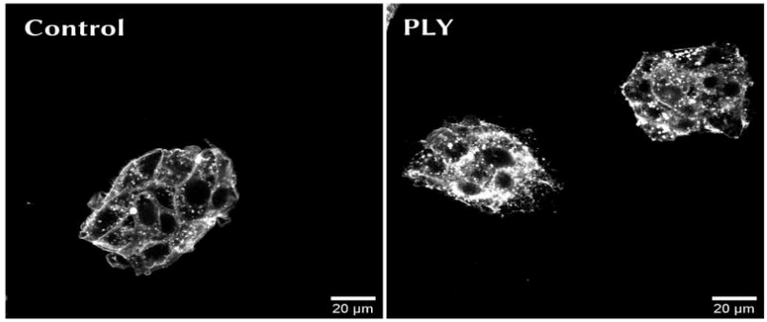
Figure: Two-photon microscopy of the Pneumolysin (PLY) induced cholesterol biosynthesis using Filipin III staining to quantify cellular sterols in HepG2 cells.
Module IV/3 - Antiviral strategies against influenza virus infection

Section of Experimental Virology
One hundred years after the most devastating pandemic of the 20th century, the Spanish flu of 1918-19, the influenza A viruses (IAV) responsible for it still pose a huge threat to public health. Seasonal IAV epidemics cause the sudden onset of respiratory problems with mild to severe disease courses. Further, IAV possess pandemic potential. Even though vaccination is the most efficient way to protect from annual IAV epidemics and new vaccines are produced every year, coverage is low, efficiency variable, and in the case of new emerging IAV subtypes, vaccinations do not provide timely protection. In addition, parts of the population reject vaccination. Licensed therapeutics in Germany are currently limited to a substance class that targets the viral neuraminidase, since the channel blockers of matrix protein 2 (M2) against IAV are no longer recommended. A major disadvantage of these antivirals is that IAV can quickly develop resistance.
In the case of an IAV infection, various cellular signaling pathways are induced, some of which are used by the virus to support its own efficient replication. Thus, alternative antiviral strategies focus on host proteins that support viral infection. In this context, repurposing of existing pharmaceutical substances targeting virus-supportive cellular factors represent promising alternatives for antiviral intervention.
During summer school participants will contribute to ongoing experiments using cellular infection models. We will investigate the activation of cellular factors upon IAV infection and the consequences of inhibition of IAV-induced cellular factors on viral replication. Sterile cell-culture work under bio-safety conditions (BSL-2) will be trained. Applied techniques include standard plaque-titration, western-blot analysis, plate-reader assays, and microscopy and/or FACS-analysis.
Module IV/4 - Experimental approaches to musculoskeletal disorders

Experimental Trauma Surgery
Musculoskeletal disorders have been the main reason for incapacitated days for years. Our group's research focuses on musculoskeletal disorders that result in dysfunction, pain and disability. Our goal is to elucidate the mechanisms that affect tissue degeneration and regeneration, with particular attention to bone, tendon and cartilage tissue. We are also interested in optimizing materials / implants to reduce the risk of material-related infections, which are serious complications in orthopedic surgery. For our studies we use human tissue and cells as well as the material from well-defined animal models. The main methods used in the various projects include gene expression analysis, protein assays, histology and immunohistochemistry, various cell culture models and a set-up for mechanical stimulation of cells in vitro. During the practical period, the student is involved in one of the ongoing projects and carries out a small project under the supervision of a team member.
- Borcherding at al. Materials 2019; doi:10.3390/ma12233838
- www.flexcellint.com
Module IV/5 - Understanding Immune dysfunction in the host response to sepsis

Center for Sepsis Control and Care
Sepsis represents a lethal syndrome caused by a dysregulated und disproportionate immune reaction to infection. The intricacies of the sepsis–associated host immune response and the often concomitant multiple organ failure (MOF) underlie high mortality (sepsis is the major cause of death in ICUs!) and the extremely limited number of treatment options. In the acute phase of sepsis, the host response often manifests as a hyper-inflammatory burst (also known as “cytokine storm”) involving the profuse release of pro-inflammatory mediators. This can cause hemodynamic complications leading to endothelial barrier dysfunction all of which can further boost immune alterations, eventually culminating in organ failure and death.
In our laboratory at the Department of Anaesthesiology and Intensive Care Medicine, we investigate the abnormalities in signal transduction responses caused by and causative for sepsis in both immune and parenchymal stromal cells. We use different approaches, e.g., in vitro model systems for organ damage or the analysis of genetic paediatric syndromes that influence the host immune and inflammatory response. In this practical course, you will analyse the biological consequences of rare pathological variants of a critical regulator of Toll receptor and inflammasome signalling discovered in rare cases of paediatric encephalopathy. We will use a combination of biochemical and flow cytometric approaches and work with genetically modified cell lines that mimic the cellular phenotypes of patients. These experiments will give you an insight into how the mechanisms of a host immune response can be studied in the laboratory using state-of-the-art technologies.
Module IV/6 - Sex differences in kidney diseases

Nephrology Lab
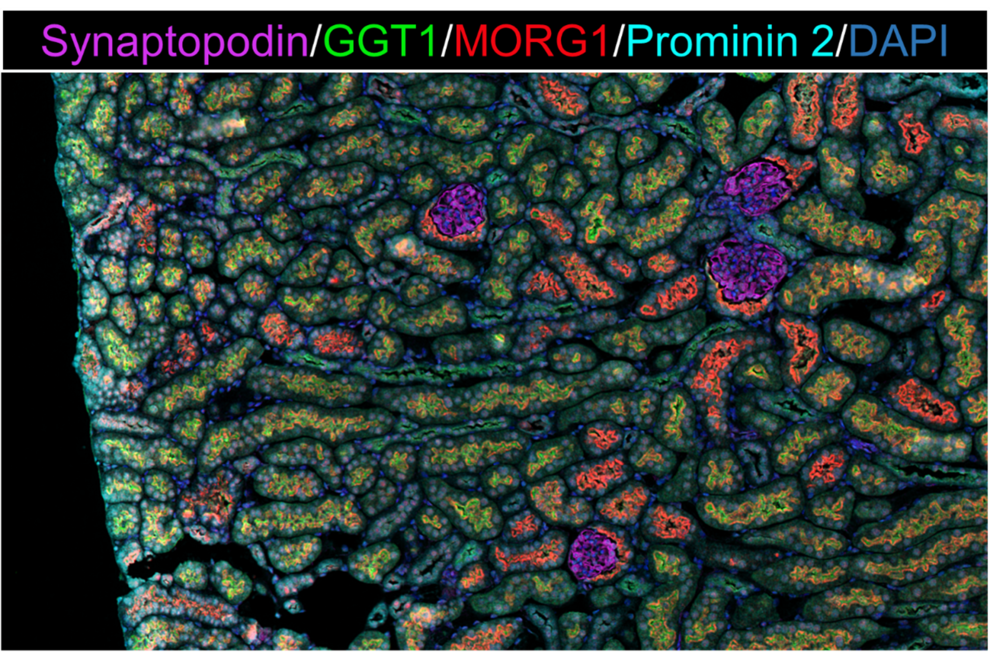
Sex differences have been described in kidney diseases such as diabetic nephropathy (DN) as well as in age-associated kidney damage. In our research group, we are mainly interested in 2 proteins that play a role in these sex differences. These are the non-fibrillar collagen type VIII and the scaffold protein MORG1, of which we know from our animal studies that their renal expressions are dependent on the factors diabetes, age and sex. In our current projects, we are trying to understand the underlying molecular mechanisms. To this end, we are working at different levels: 1) with cell lines, 2) ex vivo with pieces of kidney tissue, 3) with material from mice and 4) with human material. Our range of methods is very diverse and includes common molecular biology techniques (siRNA experiments, real-time PCR, Western blot) and various staining techniques (histochemistry, immunohistochemistry and OPAL-based multiplex immunofluorescence staining).
Module IV/7 - Metabolic adaptation during sepsis

Leibniz Institute for Natural Product Research and Infection Biology
Sepsis is a life-threatening condition characterized by dysregulated host responses to infection, frequently leading to multiple organ failure. Beyond its immunological disturbances, sepsis is accompanied by profound metabolic adaptations, which play a critical role in disease progression and recovery. The liver, as a central metabolic organ, is pivotal in coordinating systemic responses during sepsis, including glucose homeostasis, lipid metabolism, and acute-phase protein synthesis. To investigate these hepatic adaptive processes under septic conditions, we employ precision-cut liver tissue slices as an ex vivo model. This approach preserves the complex multicellular architecture of the liver and enables the analysis of metabolic, transcriptional, and functional responses to septic stimuli in a controlled environment. Our studies aim to advance the understanding of metabolic reprogramming during sepsis, providing insights that may contribute to targeted therapeutic interventions.
During summer school, students will learn how to cut organ slices, infect them and analyze protein phosphorylation and cytokine release.
![Left: Cutting organ slices with a vibratome [Siwczak, F. et al. Culture of vibrating microtome tissue slices as a 3D model in biomedical research.
J Biol Eng 17, 36 (2023)]. Right: Liver slices in our lab](/summerschoolmolmed_media/Bilder/Bild+1+und+2_Abstract+Modul+Weis/1295x527+%40+5_4-height-403-width-990-p-490.png)

