Model Project Genome Sequencing
The Healthcare Development Enhancement Act (GVWG) of July 11, 2021 (Federal Law Gazette I, p. 2754) introduced, under § 64e of the German Social Code Book V (SGB V), a model project for comprehensive diagnostics and therapy identification through genome sequencing, targeting both rare and oncological diseases. The implementation is taking place at selected clinics across Germany, including Jena University Hospital (UKJ) as the only participating site in Thuringia. The main participating institutions at UKJ are the Institute of Human Genetics (IHG), the University Tumor Center (UTC), and the Center for Rare Diseases (ZSE).
The basis of the model project is genome-wide sequencing as part of a structured clinical treatment process, with the goal of improving diagnostics, therapy, and research on rare and oncological diseases. A central component of the project is the collection and storage of clinical and genomic data in a pseudonymized data infrastructure, which has been commissioned by the project sponsor, the Federal Institute for Drugs and Medical Devices (BfArM). In the process of pseudonymization, the name or another identifying feature of a patient is replaced by a pseudonym to prevent identification of the individual involved. Great importance is always placed on the protection of personal data. Consenting for this centralized pseudonymized data storage is voluntary for all patients.
Both outpatients and inpatients with a rare or oncological disease may be included to the Model Project if they meet the criteria (see Inclusion Criteria section). Inclusion takes place through the regularly held Human Genetics Board or Molecular Tumor Boards. Prior to this inclusion, comprehensive information about the Model Project are made available to the patient and if necessary, genetic counseling. Interdisciplinary therapy planning is a central component of the model project.
The Umbrella Associations of Statutory Health Insurance Providers (GKV-SV) and Private Health Insurance Providers (PKV) have joined the agreement in accordance with Section 64e SGB V. The participating locations have an allocated annual budget for these services for the next 5 years.
In addition to the immediate benefits in terms of diagnosis and possible medical measures, patients with rare or oncological diseases will also benefit in the medium term from the improved possibilities of researching the causes and treatment options through centralized data collection.
Overview of the process
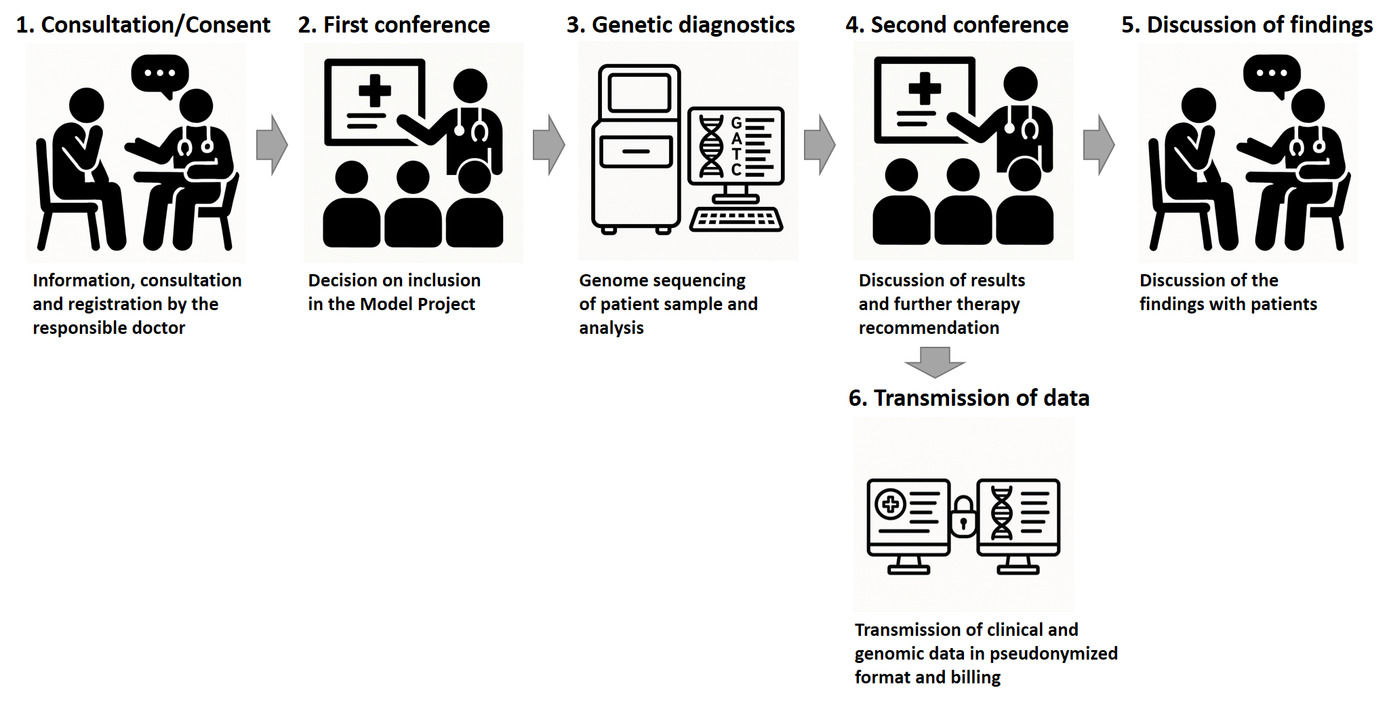
Inclusion criteria
- Existence of a medical condition: Inclusion is only possible if there is a clinical need for genome sequencing, i.e. patients with unclear, rare or complex diseases for which the available genetic and clinical examinations of standard care have not provided a diagnosis. Genome sequencing must also be expected to provide clinically relevant added value for further treatment.
- Patient consent: Patients have to give their informed consent to participate in the model project. Consent in accordance with the German Genetic Diagnostics Act is also required for examinations in the context of rare diseases and tumor predisposition syndromes. In order to be able to use the data to work on a subsequent research-based question, the release of pseudonymized data for research purposes is also requested (Broad Consent). This consent is given on a voluntary basis.
- Indication review by interdisciplinary case conference at Jena University Hospital: The Jena University Hospital holds regular interdisciplinary case conferences to discuss cases and determine whether a patient should ultimately be included in the pilot project. These interdisciplinary case conferences are made up of experts from all relevant fields with respect to the patient or case. For oncological diseases, decisions are made by the Molecular Tumor Board of the University Tumor Center Jena, and for rare diseases by the Human Genetics Board of the Institute of Human Genetics..
Forms for the application
The following declarations of consent (in German) are required to have been filled before proceeding to apply for the Model Project Genome Sequencing:
1. Consent in accordance with the Genetic Diagnosis
2. Consent to participation in the genome sequencing pilot project (participant declaration)
I. Patient Information (participant declaration)
II. Consent (participant declaration)
3. Consent to the use of patient data, health insurance data, and biomaterials for clinical research in accordance with data protection regulations (Broad Consent)
For adults
I. Patient information (adults)
For persons under age
II. Patient information (General for Minors)
III. Patient information (7-11 years)
IV. Patient Information (12-17 years)
Depending on the medical condition, application for participation is then made via the Human Genetics Board for patients with a history of a rare disease or the Molecular Tumor Board for patients with an oncological disease.
Contacts

Arbeitsgruppenleiterin



