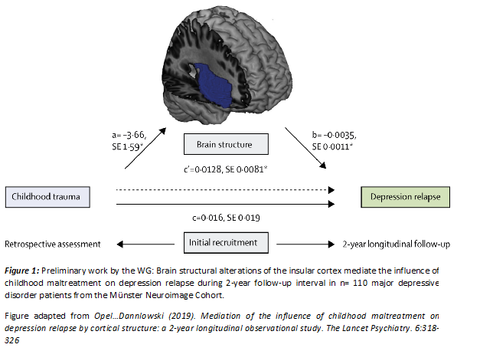
Psychiatric disorders such as major depression are among the most common disorders and represent one of the leading causes of disability worldwide. Maladaptive alterations in structure and function of neural circuits are increasingly recognized as a key mediator in the association between risk factors such as environmental stress and the development of psychiatric disorders. Despite the increasing knowledge on the neurobiological basis of psychiatric disorders, we are currently unable to estimate how successful any one course of action will be at alleviating symptoms for each individual patient. Against this backdrop, we aim (i) to generate insights on the risk factors and neurobiological mechanisms that determine individual disease trajectories with a special emphasis on affective disorders and (ii) to transfer these insights into optimized diagnostic, treatment and prevention in clinical routine. To this end, we conduct studies that employ a combination of multimodal neuroimaging (structural T1, rs-fMRI and DTI) and digitized longitudinal phenotyping in patients with affective disorders before and after different interventions (neurostimulation, pharmacology, psychotherapy). These investigations will allow to (a) disentangle neural circuit alterations in high-risk subgroups, (b) identify their value for an individual prediction of treatment response, (c) understand how interventions normalize maladaptive neural circuits to achieve remission.
During the summer school participants will be introduced to ongoing neuroimaging studies of the working group. They will thus gain insights on MR-measurements, clinical interviews, neuropsychological testing and digital phenotyping with patients. Motivated participants have the chance to work on a dedicated research question and analyze neuroimaging data under supervision of a team member.