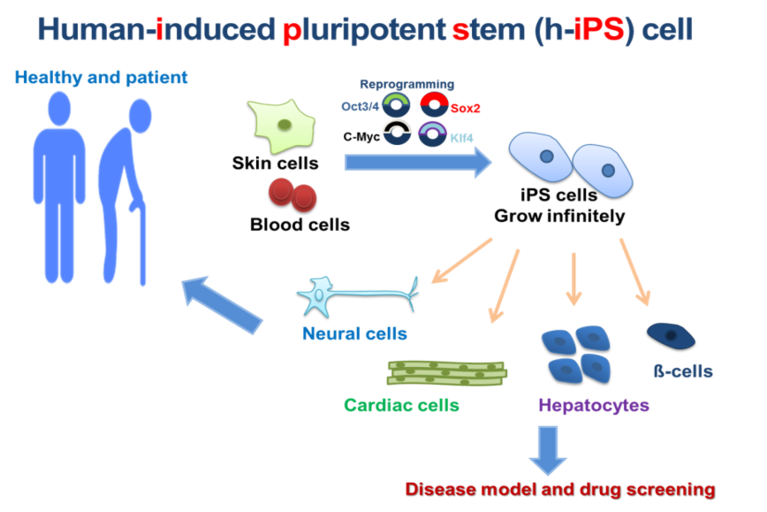
The discovery of induced pluripotent stem (iPS) cell technology has opened up unprecedented opportunities to generate patient-specific cell types in vitro, to elucidate the underlying mechanisms of physiology and pathology of the human heart. For that, we established the human-iPS cell technology as a promising generation of pluripotent stem cells using non-integrating methods to introduce the reprogramming factors (OCT4, SOX2, KLF4, and c-MYC) to the somatic cells.
The generation of human-iPS cell-derived cardiomyocytes is of growing interest for multiple applications. First, access to an in vitro model of human development permits the study of human heart development. Second, iPS cell-derived cardiomyocytes serve as a human cardiac model that can be used for diverse basic research studies ranging from cellular electrophysiology to protein biochemistry. Furthermore, human-iPS cells represent a potential source of cell-based therapies to repair and replacement of diseased or damaged tissues.
The purpose of this course to learn the basics of human-iPS cell reprogramming, iPS cell culture and differentiation of human-iPS cells into cardiovascular tissue. Therefore, we will analyze the changes in cellular and molecular of normal human-iPS cells-derived cardiovascular tissue compared with diseased tissue using different methods, such as calcium transient quantification, Seahorse metabolic profiling, gene expression, protein level, flow cytometry, morphological analysis of mitochondria, immunofluorescence staining and examined using a confocal laser scanning microscope (cLSM 900).